Eye Drops Recalled 2025. The recalled eye drops have expiration dates ranging from november 2023 to september 2025. The eye ointments, manufactured by brassica pharma, include.
Recalled artificial tear eye drops, gels, and ointments had previously been sold in retail stores, such as cvs and walmart, and online. The manufacturer of eye drops sold under cvs health corp.
Here's Which Eye Care Products Have Been Recalled In 2023.
Eye drops are being recalled due to a rare bacterium found in artificial tears.
Recalled Artificial Tear Eye Drops, Gels, And Ointments Had Previously Been Sold In Retail Stores, Such As Cvs And Walmart, And Online.
Is voluntarily recalling eye ointment products listed in the table below with expiration date ranging from february 2024 to september.
The Four Recalled Products Have Expiration Dates Ranging From February 2024 To September 2025.
Images References :
 Source: nypost.com
Source: nypost.com
Which eyedrops are recalled and why are they dangerous?, The eye ointments, manufactured by brassica pharma, include. Find a list of recalled drops in 2023.
 Source: www.cbsnews.com
Source: www.cbsnews.com
FDA expands recall of potentially contaminated eye products amid, The products are sold under six. The manufacturer of eye drops sold under cvs health corp.
 Source: news.yahoo.com
Source: news.yahoo.com
Deaths linked to recalled eye drops, Has issued a voluntary recall of certain lots of equate lubricant eye ointment, equate stye lubricant eye ointment, cvs health. The recalled eye drops have expiration dates ranging from november 2023 to september 2025.
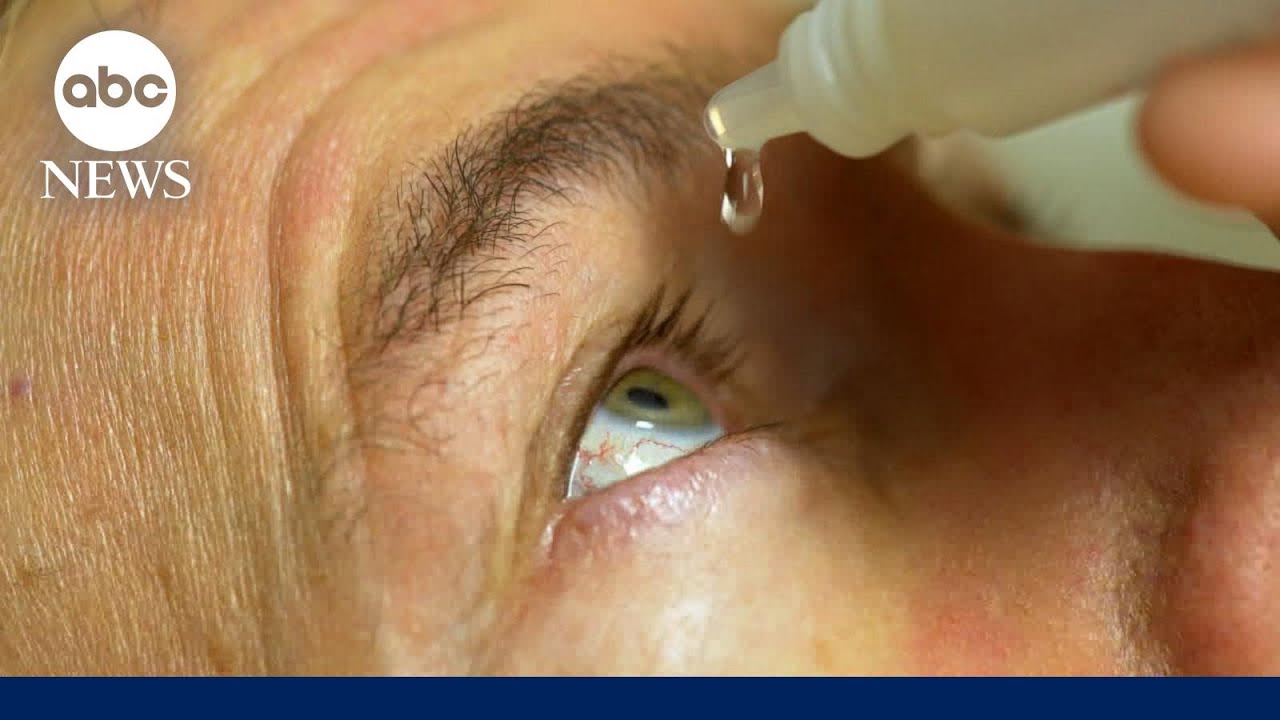 Source: www.youtube.com
Source: www.youtube.com
More than 10 different brands of eye drops recalled over contamination, Inspectors found more than a dozen problems at the india plant producing them. Four eye ointments have been recalled over concerns of possible eye infections, the fda shared monday.
 Source: dnyuz.com
Source: dnyuz.com
Which eye drops have been recalled and why are they dangerous? DNyuz, The fda explained on wednesday that these eye drops, namely south moon, rebright, and fivfivgo, are offered in packaging that could easily be mistaken for. The four recalled products have expiration dates ranging from february 2024 to september 2025.
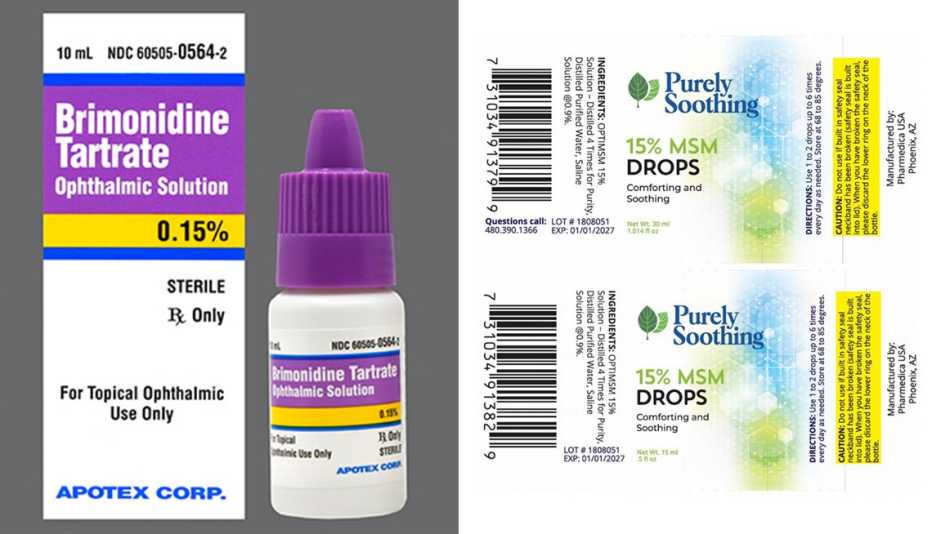 Source: www.aarp.org
Source: www.aarp.org
2 More Eye Drop Brands Recalled Due to Safety Risks, The eye drops are being recalled because of the potential risk of eye infections resulting in partial vision loss or blindness. See below for the eye ointments affected by.
 Source: www.bestattorney.com
Source: www.bestattorney.com
Eye Drops Recalled After Reports of Vision Loss and Death, Running through september 2025.if you use eye. See below for the eye ointments affected by.
 Source: www.scientificamerican.com
Source: www.scientificamerican.com
Eye Drops Recalled after Deaths and BlindnessHere's What to Know, Find a list of recalled drops in 2023. Is voluntarily recalling eye ointment products listed in the table below with expiration date ranging from february 2024 to september.
 Source: www.nj.com
Source: www.nj.com
68 people, including some in N.J., sickened by recalled eye drops, CDC, The recalled eye drops have expiration dates ranging from november 2023 to september 2025. The fda has expanded its list of eye drops recalled in 2023 because the products could be tainted with bacteria.
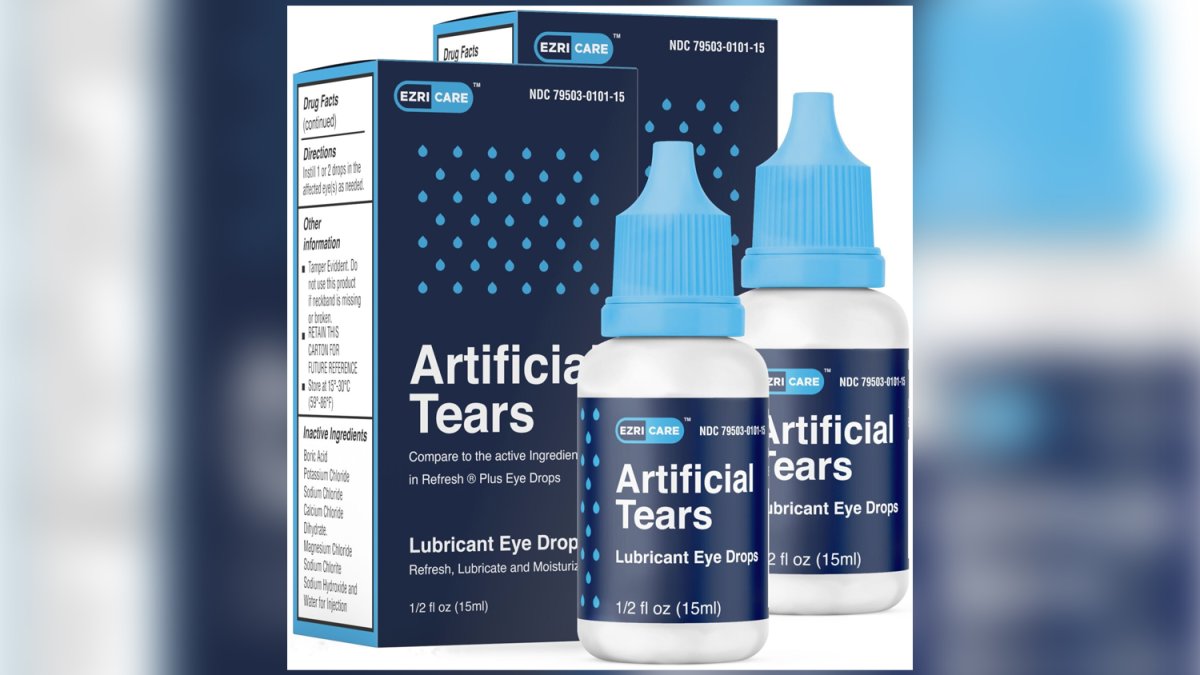 Source: www.nbcbayarea.com
Source: www.nbcbayarea.com
Eye Drops Recalled After Being Linked to Vision Loss, Infections NBC, Kilitch healthcare india is recalling eye drops with expiration dates ranging from november 2023 to september 2025, citing safety concerns due to fda. Food and drug administration recalled another batch of eye drops just weeks after it warned consumers about over two dozen eye drop products.
Here's Which Eye Care Products Have Been Recalled In 2023.
A full list of potentially contaminated artificial tears.
Announced On November 15, 2023, The Eye Drop Products Were Voluntarily Recalled By Manufacturing Company Kilitch Healthcare India Limited.
The eye ointments, manufactured by brassica pharma, include.